Expo
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.
HematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events

- Screening Tool Detects Multiple Health Conditions from Single Blood Drop
- Integrated Chemistry and Immunoassay Analyzer with Extensive Assay Menu Offers Flexibility, Scalability and Data Commutability
- Rapid Drug Test to Improve Treatment for Patients Presenting to Hospital
- AI Model Detects Cancer at Lightning Speed through Sugar Analyses
- First-Ever Blood-Powered Chip Offers Real-Time Health Monitoring
- Experimental Blood Test Improves Detection of Early-Stage Pancreatic Cancer
- Simple Blood Draw Helps Diagnose Lung Cancer 10 Times Faster
- WHO Approves First Mpox Diagnostic Test for Emergency Use
- Molecular Multiplexing Panel for Blood Culture Identification Enables Targeted Treatment Decisions
- Clinical Digital PCR System for Oncology Testing Delivers Highly Accurate Diagnostic Results
- Blood Platelet Score Detects Previously Unmeasured Risk of Heart Attack and Stroke
- Automated Benchtop System to Bring Blood Testing To Anyone, Anywhere
- New Hematology Analyzers Deliver Combined ESR and CBC/DIFF Results in 60 Seconds
- Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
- First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
- Simple Blood Test Identifies Multiple Myeloma Patients Likely to Benefit from CAR-T Immunotherapy
- Portable Device Analyzes White Blood Cell Activity to Monitor Cancer Patients’ Health
- New Test Detects Return of Blood Cancer a Year Earlier
- Universal Blood Test Could Predict Organ Transplant Outcomes with Unprecedented Accuracy
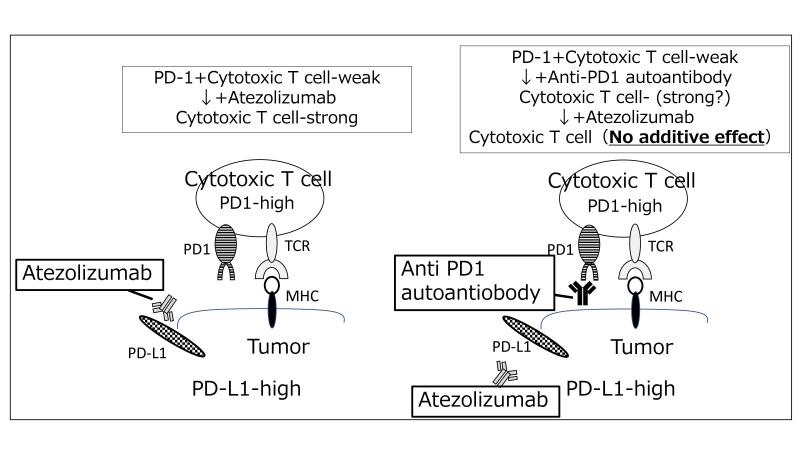
- AI Tool Predicts Cancer Patients’ Response to Immunotherapy
- New Digital PCR Assays Enable Accurate and Sensitive Detection of Critical Pathogens
- Rapid Diagnostic System to Deliver Same-Shift Antibiotic Susceptibility Test Results
- AST System Delivers Actionable Results for Gram-Negative Bacteria Directly from Positive Blood Cultures
- Ultra-Rapid Culture-Free Sepsis Test Reduces Testing Time from Days to Hours
- New Rapid Method for Determining Virus Infectivity Could Revolutionize Response to Future Pandemics
- Breath-Based Sampling System Diagnoses Lower Respiratory Tract Infection
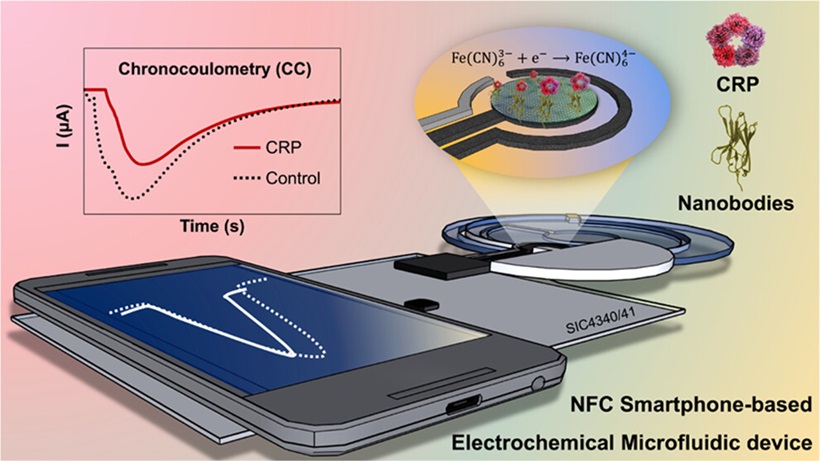
- POCT Device Monitors C-Reactive Protein Levels Associated with Inflammation in Real Time
- New Microfluidics Method to Speed Up Blood Analyses
- AI Tongue Analysis Model 98% Accurate in Detecting Diseases
- Microneedle Patch Detects Skin Cancer Early
- MEDICA LABMED FORUM 2024: International Experts Meet to Discuss Trending Topics in Laboratory Medicine
- Nova Biomedical and Terumo BCT Collaborate on Automated Cell Culture Sensing
- Beckman Coulter and Scopio Labs Add World's First Digital Bone Marrow Imaging and Analysis to Long-Term Partnership
- Roche Expands Digital Pathology Open Environment with Integration of Advanced AI Algorithms from New Collaborators
- Qiagen and Eli Lilly to Develop First QIAstat-Dx IVD Panel for Neurodegenerative Applications
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- AI Technology Accurately Predicts Breast Cancer Risk Via ‘Zombie Cells’
- Liquid Biopsy Solution Enables Non-Invasive Sample Collection and Direct Cell-Free DNA Stabilization from Urine
- Innovative AI Tool Uses Digitized Whole-Slide Images for Intermediate-Risk Prostate Management
- AI-Based Tissue Staining Detects Amyloid Deposits Without Chemical Stains or Polarization Microscopy
- Super-Resolution Imaging Detects Parkinson's 20 Years Before First Motor Symptoms Appear

Expo
 view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.
HematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
view channel
Clinical Chem.
HematologyImmunologyMicrobiologyPathologyTechnologyIndustry
Events
Advertise with Us


- Screening Tool Detects Multiple Health Conditions from Single Blood Drop
- Integrated Chemistry and Immunoassay Analyzer with Extensive Assay Menu Offers Flexibility, Scalability and Data Commutability
- Rapid Drug Test to Improve Treatment for Patients Presenting to Hospital
- AI Model Detects Cancer at Lightning Speed through Sugar Analyses
- First-Ever Blood-Powered Chip Offers Real-Time Health Monitoring
- Experimental Blood Test Improves Detection of Early-Stage Pancreatic Cancer
- Simple Blood Draw Helps Diagnose Lung Cancer 10 Times Faster
- WHO Approves First Mpox Diagnostic Test for Emergency Use
- Molecular Multiplexing Panel for Blood Culture Identification Enables Targeted Treatment Decisions
- Clinical Digital PCR System for Oncology Testing Delivers Highly Accurate Diagnostic Results
- Blood Platelet Score Detects Previously Unmeasured Risk of Heart Attack and Stroke
- Automated Benchtop System to Bring Blood Testing To Anyone, Anywhere
- New Hematology Analyzers Deliver Combined ESR and CBC/DIFF Results in 60 Seconds
- Next Generation Instrument Screens for Hemoglobin Disorders in Newborns
- First 4-in-1 Nucleic Acid Test for Arbovirus Screening to Reduce Risk of Transfusion-Transmitted Infections
- Simple Blood Test Identifies Multiple Myeloma Patients Likely to Benefit from CAR-T Immunotherapy
- Portable Device Analyzes White Blood Cell Activity to Monitor Cancer Patients’ Health
- New Test Detects Return of Blood Cancer a Year Earlier
- Universal Blood Test Could Predict Organ Transplant Outcomes with Unprecedented Accuracy
- AI Tool Predicts Cancer Patients’ Response to Immunotherapy
- New Digital PCR Assays Enable Accurate and Sensitive Detection of Critical Pathogens
- Rapid Diagnostic System to Deliver Same-Shift Antibiotic Susceptibility Test Results
- AST System Delivers Actionable Results for Gram-Negative Bacteria Directly from Positive Blood Cultures
- Ultra-Rapid Culture-Free Sepsis Test Reduces Testing Time from Days to Hours
- New Rapid Method for Determining Virus Infectivity Could Revolutionize Response to Future Pandemics
- Breath-Based Sampling System Diagnoses Lower Respiratory Tract Infection
- POCT Device Monitors C-Reactive Protein Levels Associated with Inflammation in Real Time
- New Microfluidics Method to Speed Up Blood Analyses
- AI Tongue Analysis Model 98% Accurate in Detecting Diseases
- Microneedle Patch Detects Skin Cancer Early
- MEDICA LABMED FORUM 2024: International Experts Meet to Discuss Trending Topics in Laboratory Medicine
- Nova Biomedical and Terumo BCT Collaborate on Automated Cell Culture Sensing
- Beckman Coulter and Scopio Labs Add World's First Digital Bone Marrow Imaging and Analysis to Long-Term Partnership
- Roche Expands Digital Pathology Open Environment with Integration of Advanced AI Algorithms from New Collaborators
- Qiagen and Eli Lilly to Develop First QIAstat-Dx IVD Panel for Neurodegenerative Applications
- Gene Panel Predicts Disease Progession for Patients with B-cell Lymphoma
- New Method Simplifies Preparation of Tumor Genomic DNA Libraries
- New Tool Developed for Diagnosis of Chronic HBV Infection
- Panel of Genetic Loci Accurately Predicts Risk of Developing Gout
- Disrupted TGFB Signaling Linked to Increased Cancer-Related Bacteria
- AI Technology Accurately Predicts Breast Cancer Risk Via ‘Zombie Cells’
- Liquid Biopsy Solution Enables Non-Invasive Sample Collection and Direct Cell-Free DNA Stabilization from Urine
- Innovative AI Tool Uses Digitized Whole-Slide Images for Intermediate-Risk Prostate Management
- AI-Based Tissue Staining Detects Amyloid Deposits Without Chemical Stains or Polarization Microscopy
- Super-Resolution Imaging Detects Parkinson's 20 Years Before First Motor Symptoms Appear


















.jpg)










 (1)_2.jpg)